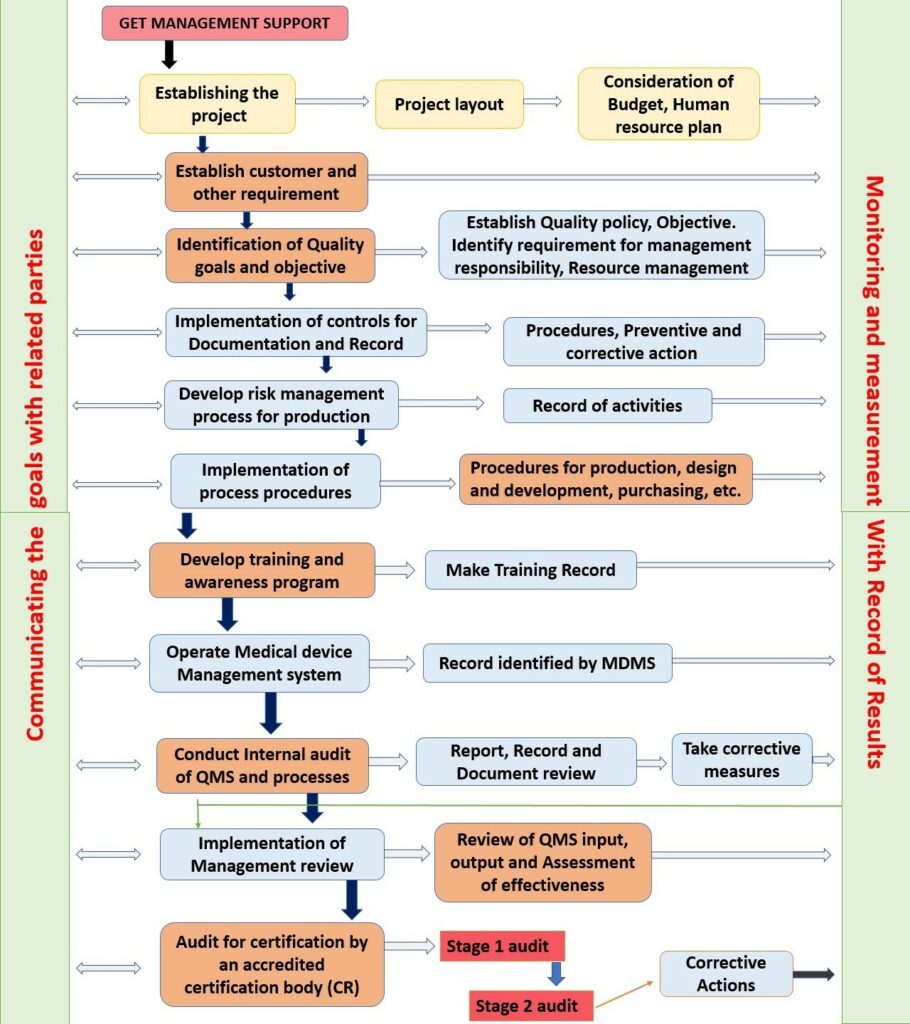
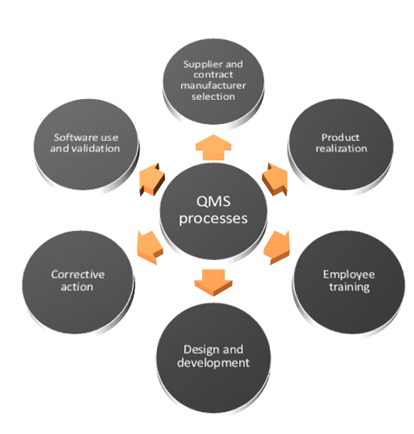
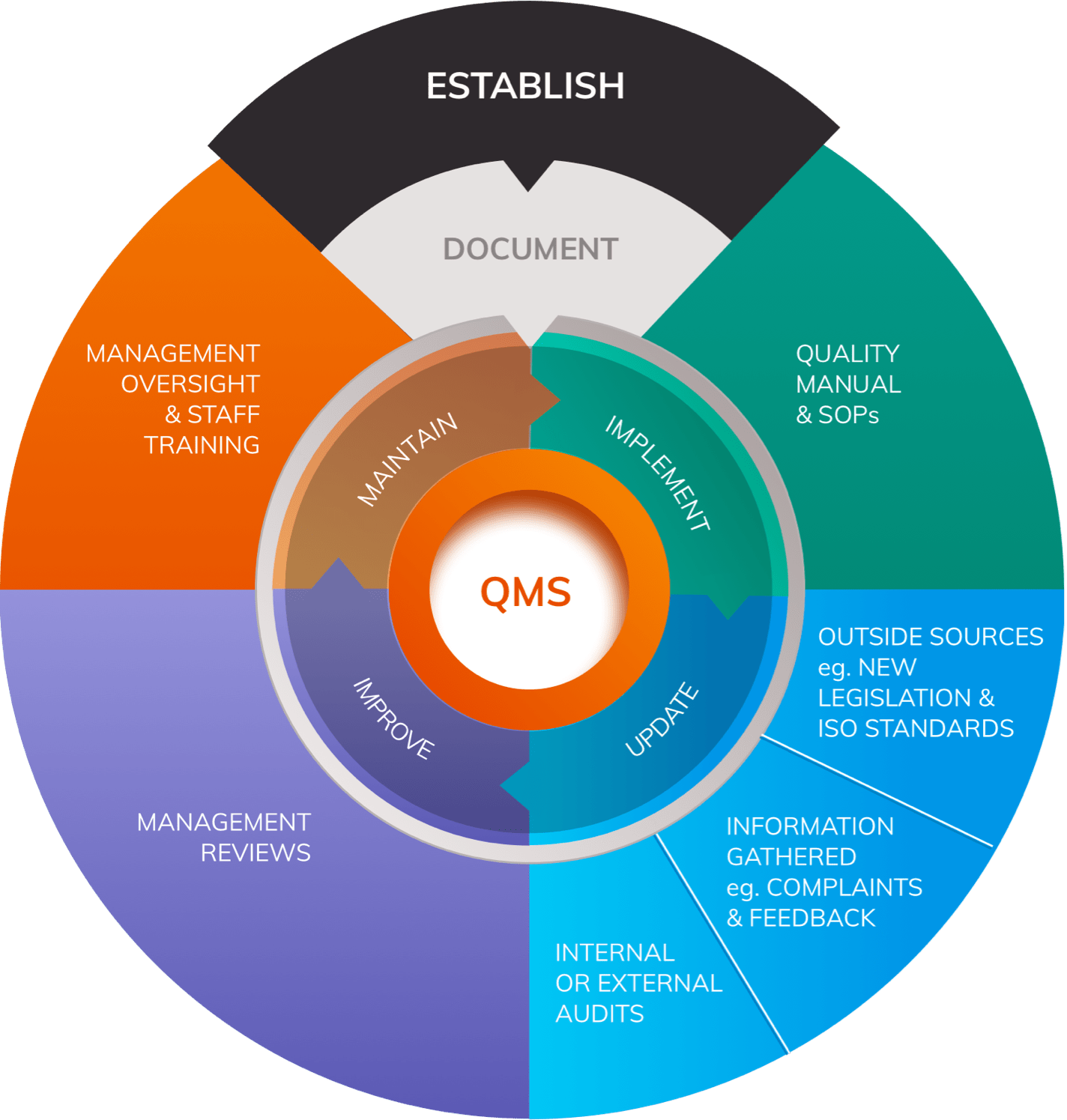
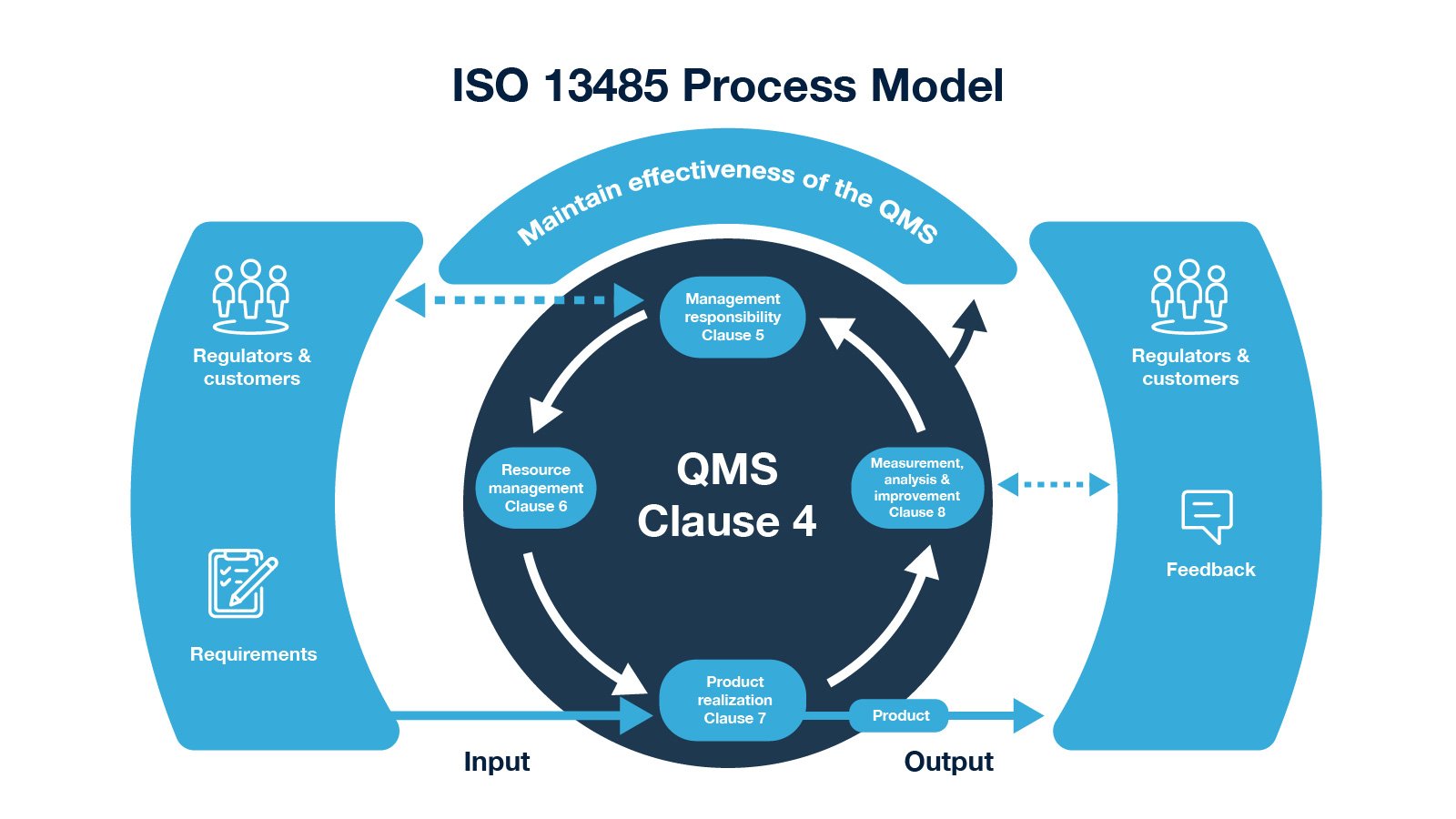
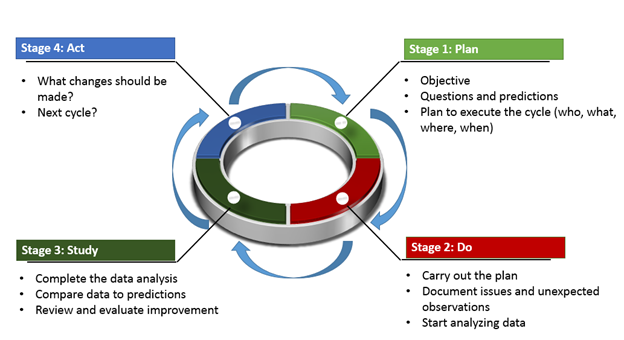
ISO 13485: Basics and How to Get Started (QMS for Medical Devices) | Process Street | Checklist, Workflow and SOP Software
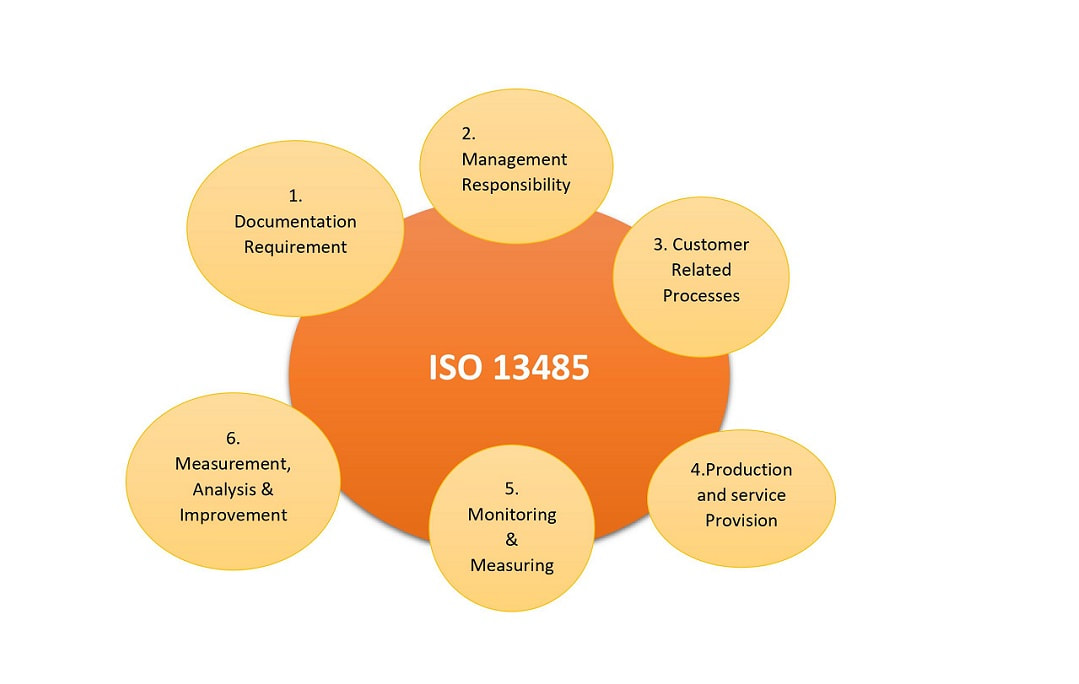
ISO 13485 Quality Management System for Medical Devices Certification Consulting - GlenView Group, Inc. - ISO Lead Auditor Certification Courses - Consulting & Training

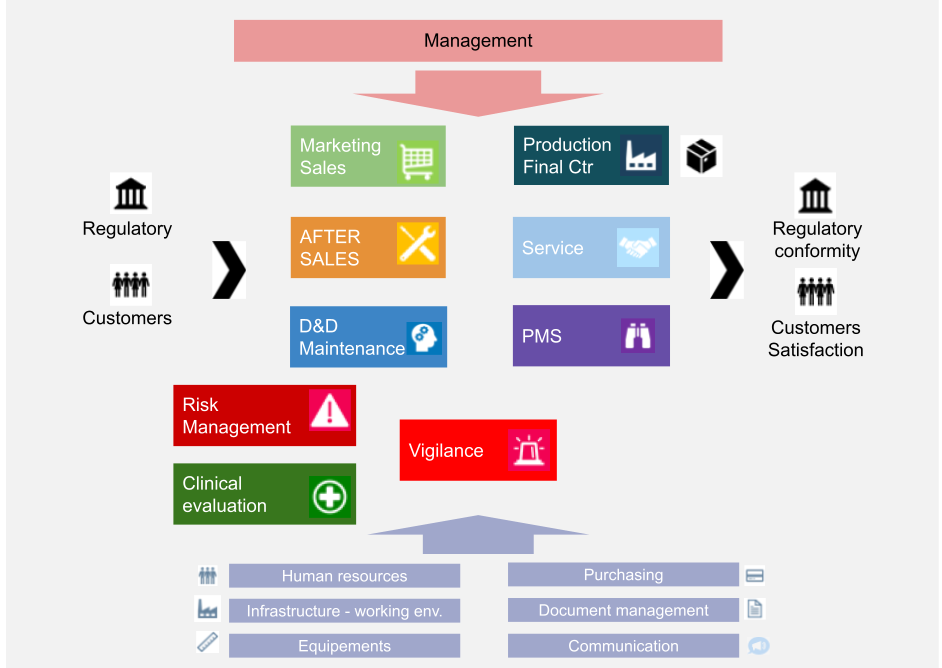
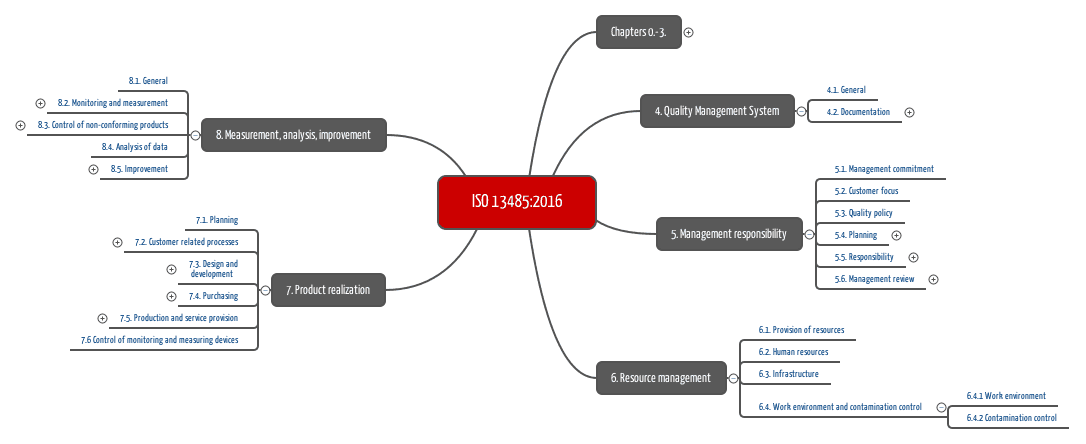



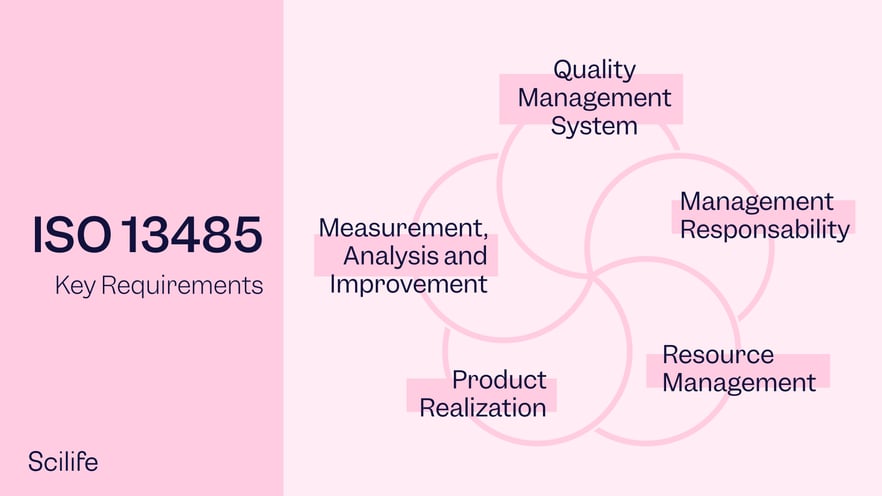

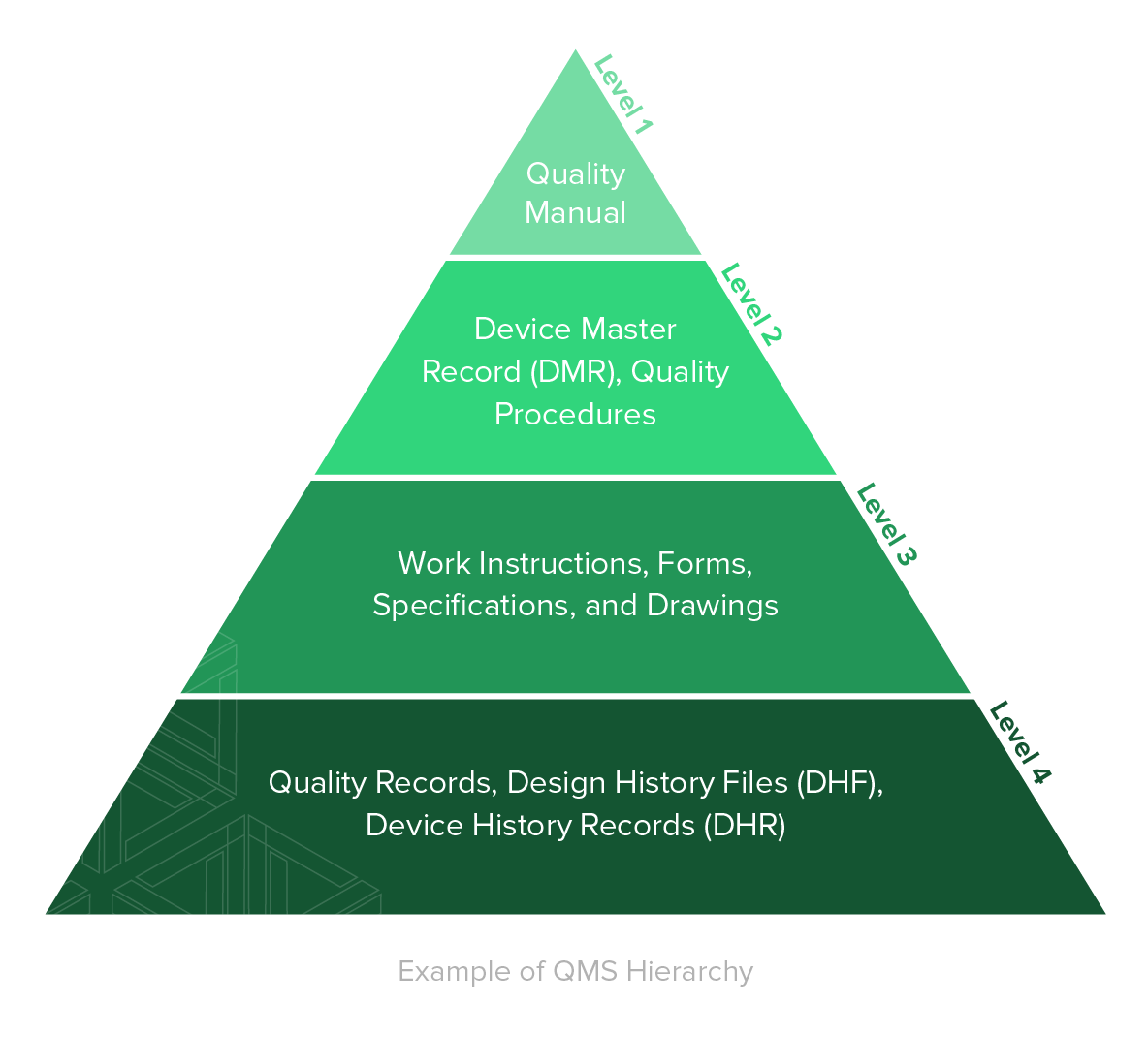

.jpg)